Inside Kyron.bio’s Bold Bet to Rewrite the Rules of Antibody Drug Design
For all the buzz around antibody therapies, one overlooked culprit keeps holding them back: sugars. Paris-based Kyron.bio is tackling the hidden complexity of glycosylation — the way sugars attach to therapeutic proteins — with a platform that promises safer, longer-lasting drugs.

Latest

La Machine #47: Superbugs, Beats, and Billions
Phagos uses AI-designed bacteriophages to fight bacterial infections; Claimy is building algorithms to chase lost musical royalties. Plus: Mistral AI gets a fancy new crib; Paris ranks #3 in global AI hubs; Probabl leads AI funding with €13M round.
Claimy Wants to Use AI to End Music’s Missing Money Problem
Claimy, a Paris startup backed by €1.5 million in pre-seed funding, is building an AI-powered platform to locate and reclaim lost royalties, attacking a $15 billion global problem that’s eroded transparency and trust in the booming music economy.
How Phagos Plans to Kill Superbugs with AI and Nature’s Own Viruses
Antibiotics are failing. French biotech Phagos thinks the answer lies in nature’s own bacteria-killers: 'phages.' With €25 million and an AI platform to match viruses with their prey, the startup could rewrite the rulebook on how we fight infection.
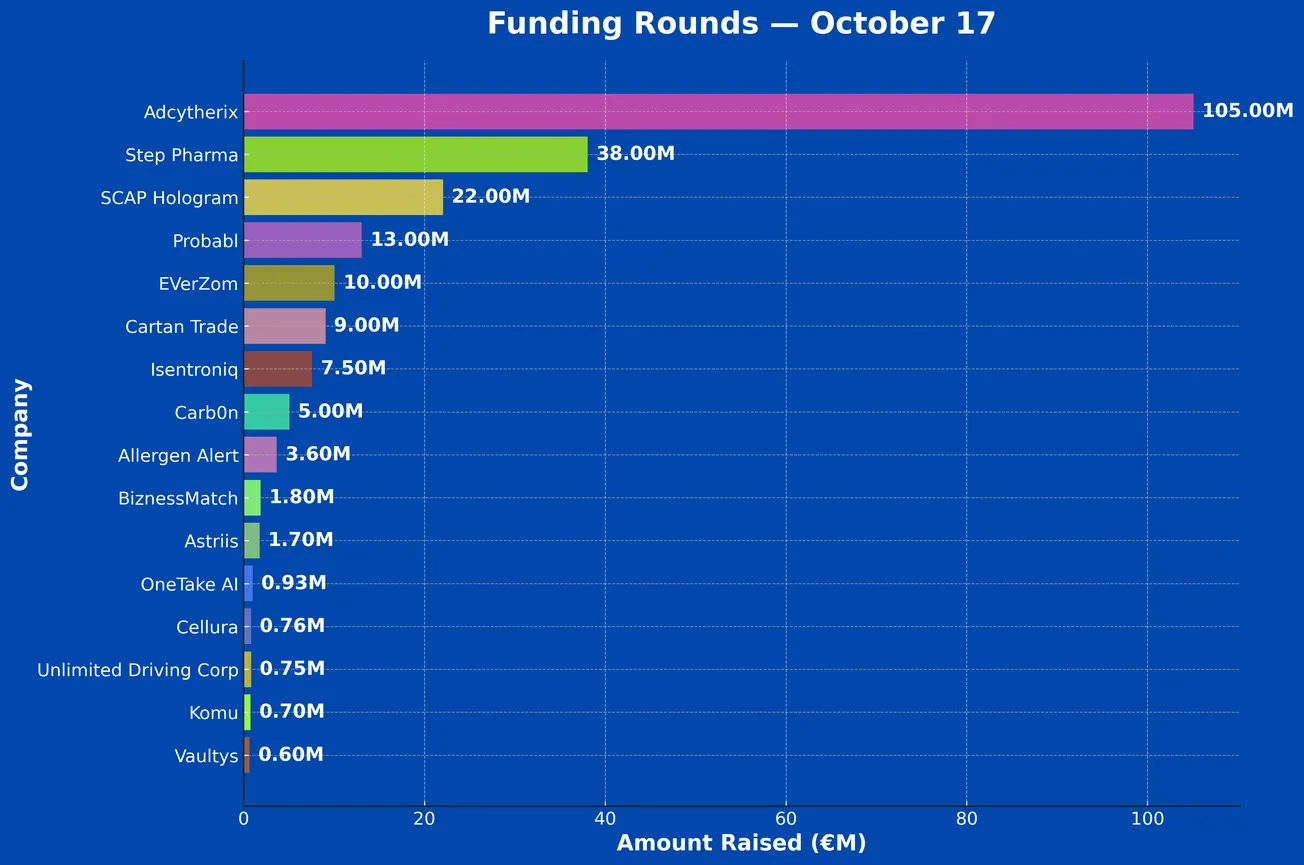
French Tech Funding Wire Oct 17: The Big $300M Deel Deal; Plus Adcytherix, Step Pharma, SCAP Hologram & More!
Between Oct 13 and Oct 17 a total of 16 French Tech companies raised €225.28 million, including: Probabl, EVerZom, Cartan Trade, Isentroniq, Carb0n, Allergen Alert, BiznessMatch, Astriis, OneTake AI, Cellura, Unlimited Driving Corporation (UDC), Vaultys, and Komu.




