MSInsight : Paving the Path towards Precision Oncology
CEO and co-founder Arnaud Dr. Cutivet: “MSI cancer cells are present in 4% of cancers, especially colorectal, stomach, and endometrial cancers. Early detection, particularly in colorectal cancer, means we can use immunotherapy effectively, potentially saving lives.”
Latest
How Cybersecurity Startup MokN Combats Phishing With Deceptive 'Fake Doors'
When stolen credentials breached his 'impenetrable' system, Gautier Bugeon had an insight: give attackers fake doors to try. His startup MokN raised €2.6M, hit $1M ARR in 16 months with half its clients being billion-dollar companies, and is now expanding to America.

When Bots Go Agentic: How AI Is Rewriting the Rules of Cybersecurity
The cyber arms race has accelerated: humans once led attacks, AI now augments them, and MOAIs may soon strike autonomously. The big question: can defenders evolve fast enough to match a threat that never sleeps and learns on the fly?
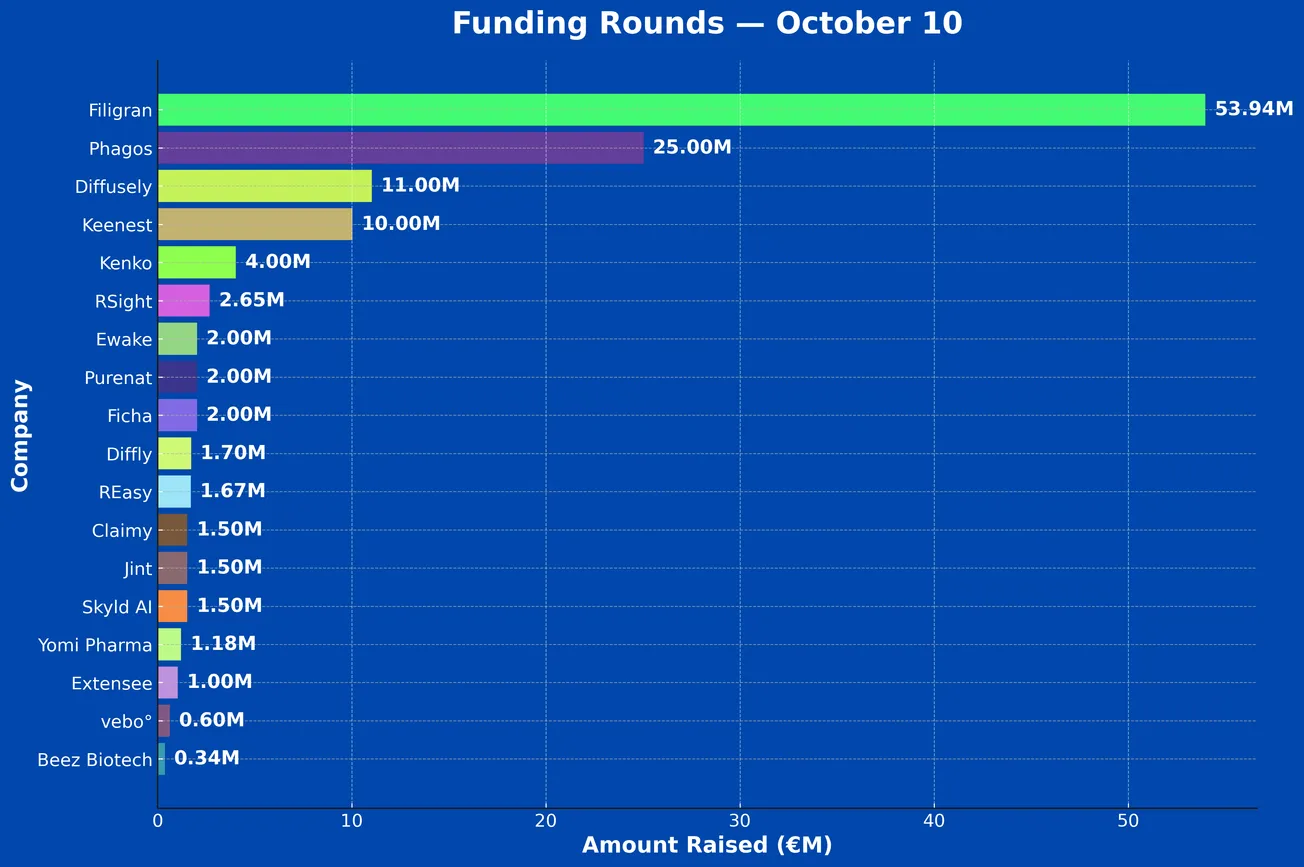
French Tech Funding Wire Oct 10: Filigran, Phagos, Diffusely & More!
Between Oct 6 and Oct 10 a total of 19 French Tech companies raised €123.6M including: Diffusely, Keenest, Kenko, RSight, Ewake, Purenat, Ficha, Diffly, Claimy, Jint, Skyld AI, Yomi Pharma, Extensee, vebo°, REasy, Beez Biotech, Biomunex Pharmaceuticals.

🛡️Special Edition: French Cybersecurity Takes Center Stage
France bets big on cybersecurity as global leaders gather in Monaco. Part 1 of our special edition maps the nation’s €1B cyber strategy—spanning government, defense, startups, and AI-driven innovation—while revealing a widening gap between policy promises and on-the-ground protection.